As a gas occupies 900.0 mL, we embark on an intriguing journey into the captivating world of gases. Prepare to be enthralled by the interplay of volume, pressure, and temperature as we unravel the mysteries that govern these elusive substances.
From the fundamental properties of gases to the profound implications of gas laws, this exploration promises to illuminate the intricate workings of gases and their pervasive influence in diverse scientific fields.
Properties of Gases
A gas is a state of matter characterized by low density and high fluidity. Unlike solids and liquids, gases do not have a definite shape or volume. They expand to fill the container they are in and can flow easily.
The properties of gases are largely determined by their volume, pressure, and temperature. These three variables are related by the ideal gas law, which states that the product of the pressure and volume of a gas is proportional to its temperature.
Volume
The volume of a gas is the amount of space it occupies. Gases can be compressed or expanded, which changes their volume. When a gas is compressed, its volume decreases and its pressure increases. Conversely, when a gas is expanded, its volume increases and its pressure decreases.
Pressure
The pressure of a gas is the force exerted by the gas on the walls of its container. Gases exert pressure in all directions. The pressure of a gas is proportional to its temperature and volume. When the temperature of a gas increases, its pressure also increases.
A gas occupies 900.0 mL, and its properties are worth exploring. Like the posture of qtip is , it can change its shape and volume depending on its surroundings. However, the gas’s volume of 900.0 mL remains constant, making it a subject of further investigation.
Similarly, when the volume of a gas decreases, its pressure increases.
Temperature, A gas occupies 900.0 ml
The temperature of a gas is a measure of the average kinetic energy of its molecules. Gases with higher temperatures have molecules that move faster and have more energy. The temperature of a gas is proportional to its pressure and volume.
When the temperature of a gas increases, its pressure and volume also increase.
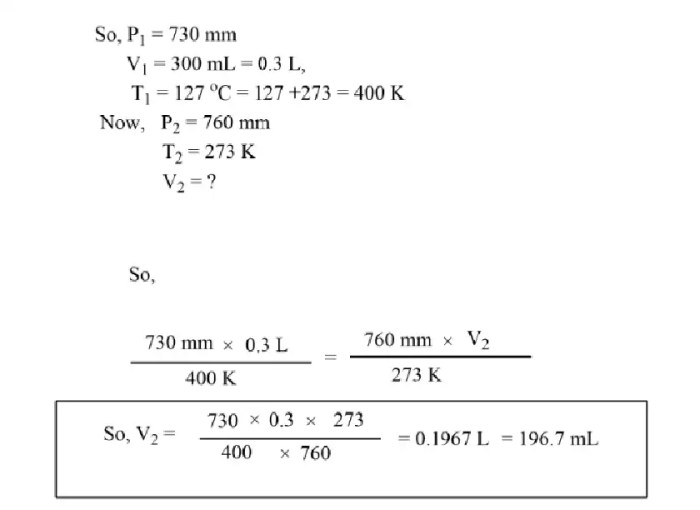
Boyle’s Law

Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. As pressure increases, volume decreases, and vice versa. This relationship can be expressed mathematically as P₁V₁ = P₂V₂, where P₁ and V₁ represent the initial pressure and volume, and P₂ and V₂ represent the final pressure and volume.
Inverse Relationship between Pressure and Volume
The following table demonstrates the inverse relationship between pressure and volume:| Pressure (kPa) | Volume (mL) ||—|—|| 100 | 900 || 200 | 450 || 300 | 300 || 400 | 225 || 500 | 180 |
Real-World Example
A simple illustration of Boyle’s Law is the behavior of a balloon. When you inflate a balloon, you increase the pressure inside it. As a result, the volume of the balloon increases. Conversely, when you release the air from the balloon, the pressure inside decreases, and the balloon shrinks in volume.
Charles’s Law

Charles’s Law, also known as the Law of Volumes, describes the relationship between the volume and temperature of a gas. It states that the volume of an ideal gas is directly proportional to its absolute temperature under constant pressure.
Mathematically, Charles’s Law can be expressed as:
V/T = constant
where: – V is the volume of the gas – T is the absolute temperature in Kelvin
Relationship between Temperature and Volume
Charles’s Law demonstrates a direct relationship between temperature and volume. As the temperature of a gas increases, its volume also increases, assuming constant pressure. Conversely, as the temperature decreases, the volume of the gas decreases.
| Temperature (K) | Volume (mL) |
|---|---|
| 273.15 | 1000 |
| 283.15 | 1035 |
| 293.15 | 1070 |
| 303.15 | 1105 |
Practical Application
Charles’s Law has numerous practical applications. One notable example is in hot air balloons. As the air inside the balloon is heated, its volume increases, causing the balloon to rise. Similarly, in scuba diving, Charles’s Law helps determine the volume of gas a diver exhales at different depths, allowing for safe and efficient decompression.
Combined Gas Law
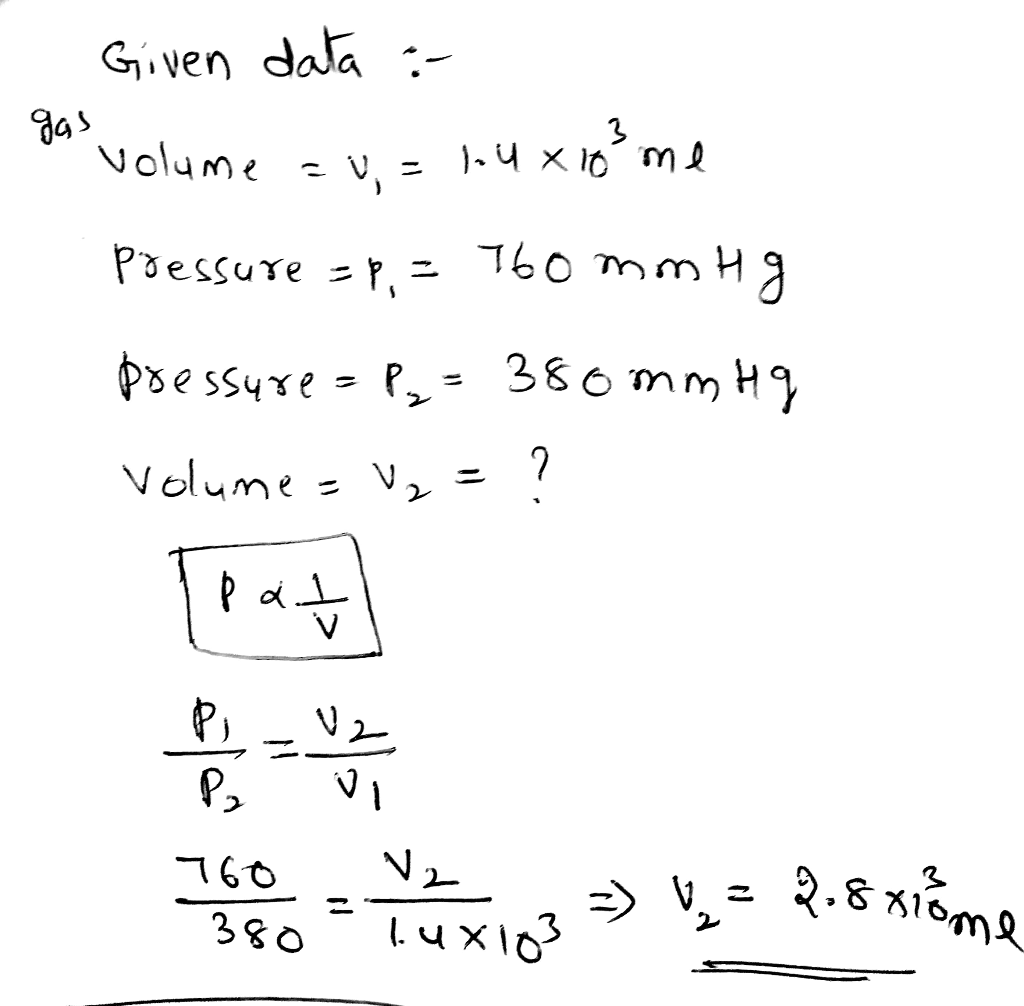
The Combined Gas Law combines Boyle’s Law and Charles’s Law into a single equation that can be used to solve problems involving changes in pressure, volume, and temperature of a gas.
The Combined Gas Law states that the product of the pressure and volume of a gas is directly proportional to the temperature of the gas. Mathematically, this can be expressed as:
P1V 1/T 1= P 2V 2/T 2
where:
- P 1is the initial pressure of the gas
- V 1is the initial volume of the gas
- T 1is the initial temperature of the gas
- P 2is the final pressure of the gas
- V 2is the final volume of the gas
- T 2is the final temperature of the gas
This equation can be used to solve a variety of problems, such as:
- Calculating the pressure of a gas when the volume and temperature change
- Calculating the volume of a gas when the pressure and temperature change
- Calculating the temperature of a gas when the pressure and volume change
Step-by-Step Guide to Using the Combined Gas Law
- Identify the known and unknown variables in the problem.
- Write down the Combined Gas Law equation: P1V 1/T 1= P 2V 2/T 2.
- Substitute the known values into the equation.
- Solve the equation for the unknown variable.
- Check your answer to make sure it makes sense.
Ideal Gas Law: A Gas Occupies 900.0 Ml
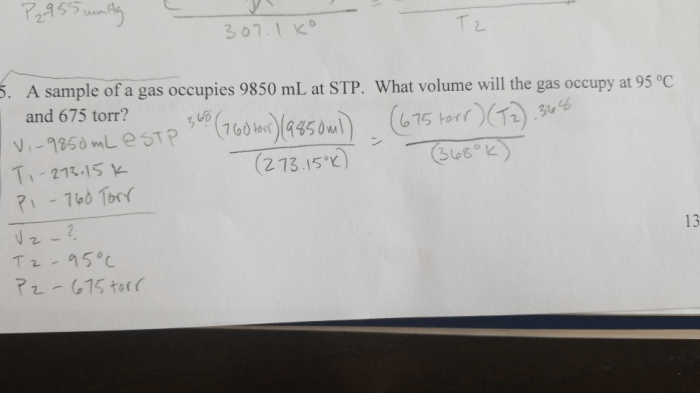
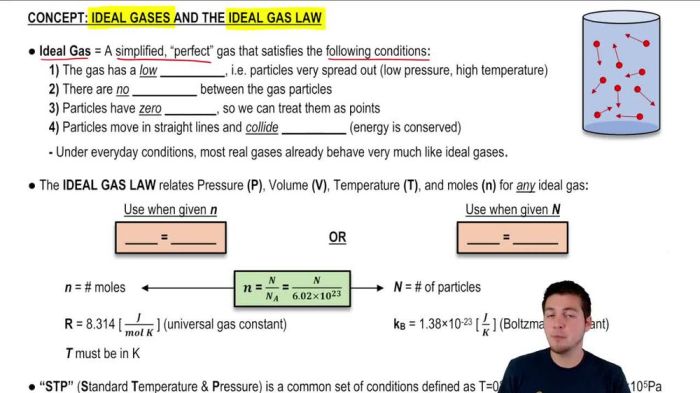
The Ideal Gas Law is a mathematical equation that describes the relationship between the pressure, volume, temperature, and number of moles of a gas. It is a combination of Boyle’s Law, Charles’s Law, and Avogadro’s Law.
The Ideal Gas Law equation is:
PV = nRT
where:
- P is the pressure of the gas in pascals (Pa)
- V is the volume of the gas in cubic meters (m³)
- n is the number of moles of the gas in moles (mol)
- R is the ideal gas constant, which is 8.314 J/(mol·K)
- T is the temperature of the gas in kelvins (K)
The Ideal Gas Law can be used to calculate any of the four variables if the other three are known. For example, if you know the pressure, volume, and temperature of a gas, you can use the Ideal Gas Law to calculate the number of moles of gas present.
The Ideal Gas Law is only accurate for ideal gases. Ideal gases are gases that obey the assumptions of the kinetic molecular theory. These assumptions are:
- The gas particles are in constant random motion.
- The gas particles are perfectly elastic and do not interact with each other.
- The gas particles occupy negligible volume compared to the volume of the container.
Real gases deviate from ideal behavior at high pressures and low temperatures. This is because the assumptions of the kinetic molecular theory break down under these conditions.
Applications of Gas Laws
Gas laws are fundamental principles that describe the behavior of gases under various conditions. They have wide-ranging applications across scientific fields, engineering, and everyday life. Understanding these laws is crucial for predicting and controlling the behavior of gases in different scenarios.
Chemistry
In chemistry, gas laws play a vital role in understanding chemical reactions and processes. For instance, Boyle’s Law helps determine the relationship between gas volume and pressure, which is essential in gas collection and analysis. Charles’s Law describes the relationship between gas volume and temperature, which is crucial in understanding reactions involving gases at different temperatures.
Physics
In physics, gas laws are used to explain phenomena such as gas expansion, compression, and pressure changes. For example, the Combined Gas Law combines Boyle’s Law and Charles’s Law to describe the behavior of gases under simultaneous changes in volume, pressure, and temperature.
This law is used in fields such as meteorology and thermodynamics.
Engineering
In engineering, gas laws are applied in various fields, including automotive engineering, aerospace engineering, and chemical engineering. For instance, the Ideal Gas Law is used to design engines, compressors, and other systems that involve gas flow. Understanding gas laws is crucial for optimizing performance, safety, and efficiency in these systems.
Case Study: Scuba Diving
Scuba diving is a real-world application where gas laws are essential. Divers must understand how gas pressure and volume change with depth to ensure their safety. Boyle’s Law helps divers calculate the volume of air they breathe at different depths, while Henry’s Law describes the absorption of gases into the bloodstream, which can lead to decompression sickness if not managed properly.
FAQ Summary
What is the significance of a gas occupying a specific volume?
The volume occupied by a gas is a crucial parameter that influences its behavior and properties. It provides insights into the gas’s density, compressibility, and interactions with its surroundings.
How do gas laws help us understand the behavior of gases?
Gas laws, such as Boyle’s Law, Charles’s Law, and the Combined Gas Law, establish quantitative relationships between the pressure, volume, and temperature of gases. These laws allow us to predict and manipulate the behavior of gases under varying conditions.
What are some practical applications of gas laws?
Gas laws find widespread applications in diverse fields. In chemistry, they aid in determining the molar mass of gases and predicting reaction yields. In physics, they contribute to understanding atmospheric pressure and fluid dynamics. In engineering, they guide the design of engines, turbines, and refrigeration systems.