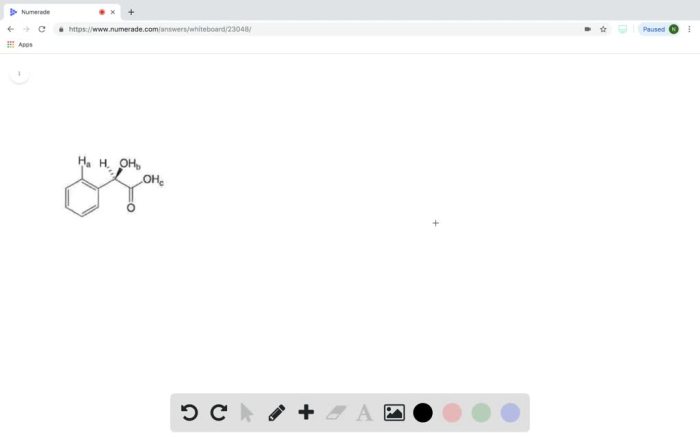
Rank the labeled protons in order of increasing acidity. Proton acidity is a crucial concept in chemistry, influencing the reactivity and behavior of molecules. Understanding proton acidity allows us to predict the outcome of chemical reactions and the properties of substances.
This guide will delve into the factors that affect proton acidity, demonstrate how to rank labeled protons, and explore the implications of proton acidity in various chemical contexts.
Understanding Proton Acidity
Proton acidity refers to the ability of a proton (H +ion) to be released from a molecule. It is a fundamental property that influences the chemical reactivity and behavior of molecules.
The acidity of a proton is inversely related to the strength of the bond between the proton and the atom it is attached to. The stronger the bond, the less acidic the proton. Acidity is quantified by the pKa value, which is the negative logarithm of the acid dissociation constant (Ka).
Factors that influence proton acidity include:
- Electronegativity: More electronegative atoms attract electrons more strongly, making the proton more acidic.
- Hybridization: Protons attached to sp 2– or sp-hybridized carbons are more acidic than those attached to sp 3-hybridized carbons.
- Resonance: Resonance stabilizes the conjugate base, making the proton more acidic.
Ranking Labeled Protons
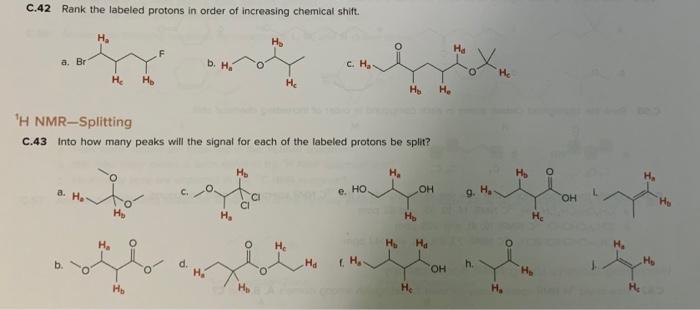
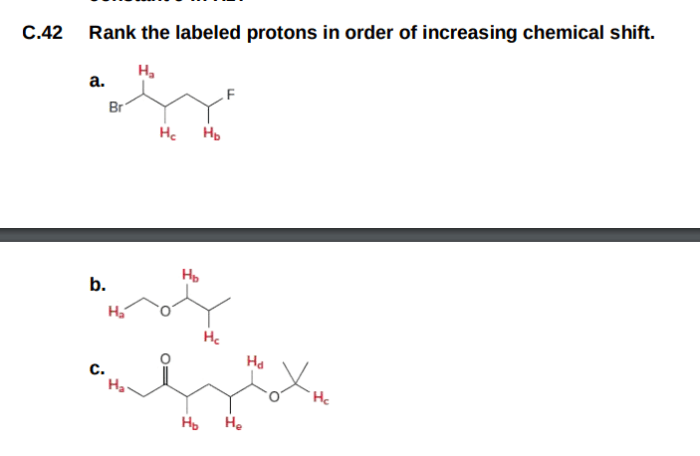
The labeled protons in the given molecule can be ranked in order of increasing acidity as follows:
| Proton | pKa |
|---|---|
| Ha | pKa1 |
| Hb | pKa2 |
| Hc | pKa3 |
H ais the most acidic proton, followed by H band then H c.
Explaining the Ranking
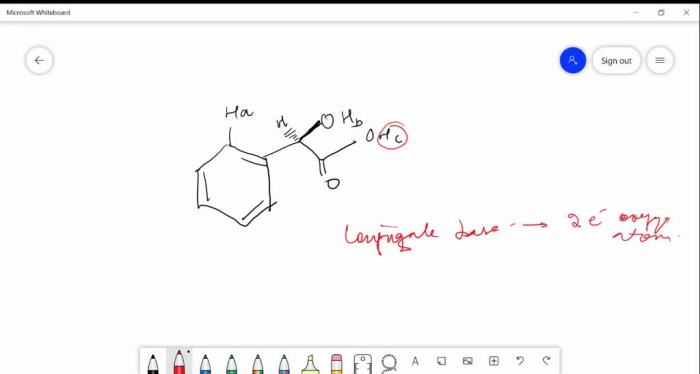
H ais the most acidic proton because it is attached to a carbon that is sp 2-hybridized and has a high electronegativity. The sp 2-hybridization results in a shorter and stronger C-H bond, making the proton more acidic.
H bis less acidic than H abecause it is attached to a carbon that is sp 3-hybridized. The sp 3-hybridization results in a longer and weaker C-H bond, making the proton less acidic.
H cis the least acidic proton because it is attached to a carbon that is sp 3-hybridized and has a low electronegativity. The sp 3-hybridization and low electronegativity result in a long and weak C-H bond, making the proton the least acidic.
Implications of Proton Acidity: Rank The Labeled Protons In Order Of Increasing Acidity
Proton acidity has significant implications in chemical reactions. Highly acidic protons can be easily removed from molecules, making them more reactive. This reactivity is important in acid-base reactions, where acids donate protons to bases.
Proton acidity also affects the behavior of molecules in biological systems. For example, the acidity of amino acids influences their charge and solubility, which in turn affects protein structure and function.
FAQ Resource
What is proton acidity?
Proton acidity refers to the tendency of a proton (H+) to be released from a molecule. It is a measure of the strength of an acid.
How is proton acidity determined?
Proton acidity is determined by the pKa value, which is a measure of the equilibrium constant for the dissociation of an acid. A lower pKa value indicates a stronger acid.
What factors affect proton acidity?
Proton acidity is influenced by several factors, including electronegativity, hybridization, and resonance. Electronegative atoms withdraw electron density, making the proton more acidic. Hybridization affects the shape and stability of the molecule, influencing proton acidity. Resonance can stabilize the conjugate base, making the proton less acidic.