Hydrogen peroxide decomposes spontaneously to yield water and oxygen, a reaction that has garnered significant scientific and practical interest. This decomposition process, characterized by the release of oxygen gas, holds immense significance in various fields, ranging from industrial applications to medical practices.
The decomposition of hydrogen peroxide is a complex chemical reaction that involves the breaking down of hydrogen peroxide molecules into water and oxygen. This process can occur spontaneously or be catalyzed by the presence of certain substances. The rate of decomposition is influenced by several factors, including temperature, concentration, and the presence of catalysts.
Hydrogen Peroxide Decomposition Overview: Hydrogen Peroxide Decomposes Spontaneously To Yield Water And Oxygen
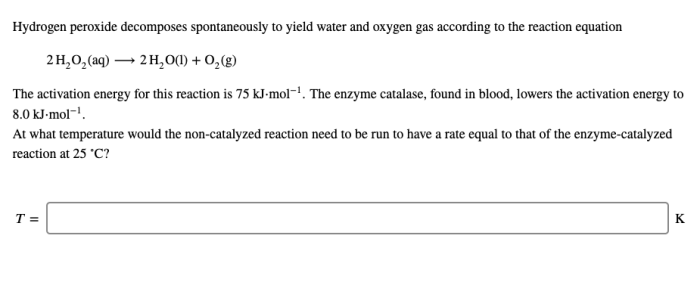
Hydrogen peroxide (H 2O 2) is a colorless liquid that decomposes spontaneously into water (H 2O) and oxygen (O 2). The chemical equation for this decomposition reaction is:
H2O 2→ 2H 2O + O 2
During decomposition, the physical changes include the formation of gas bubbles (oxygen) and a decrease in the volume of the solution. The chemical changes involve the breaking of the O-O bond in hydrogen peroxide and the formation of new O-H bonds in water and O-O bonds in oxygen.
The rate of decomposition is influenced by several factors, including temperature, pH, and the presence of catalysts.
Mechanism of Decomposition
Hydrogen peroxide decomposition proceeds via a free radical chain mechanism. The initiation step involves the homolytic cleavage of the O-O bond in hydrogen peroxide, producing two hydroxyl radicals (·OH).
The propagation steps involve the reaction of hydroxyl radicals with hydrogen peroxide, producing water and additional hydroxyl radicals. These hydroxyl radicals can then react with more hydrogen peroxide, continuing the chain reaction.
The termination steps involve the reaction of two hydroxyl radicals to form water, or the reaction of a hydroxyl radical with an antioxidant to form a stable product.
Catalysts, such as metal ions, can accelerate the decomposition process by providing an alternative pathway for the initiation step.
Applications of Hydrogen Peroxide Decomposition
Hydrogen peroxide decomposition has numerous industrial applications, including:
- Bleaching: Hydrogen peroxide is used as a bleaching agent in the textile, paper, and food industries.
- Disinfection: Hydrogen peroxide is used as a disinfectant in the medical and food industries.
- Wastewater treatment: Hydrogen peroxide is used to oxidize organic pollutants in wastewater.
Hydrogen peroxide is also used as a propellant and fuel source in rocketry and other applications.
In medicine, hydrogen peroxide is used as a wound cleaning agent and antiseptic solution.
Safety Considerations
Hydrogen peroxide decomposition can be hazardous if not handled properly. Hydrogen peroxide is a strong oxidizing agent and can cause skin irritation, eye damage, and respiratory problems.
Safety guidelines for handling and storing hydrogen peroxide include:
- Wear protective clothing, including gloves, goggles, and a lab coat.
- Store hydrogen peroxide in a cool, dark place away from flammable materials.
- Do not mix hydrogen peroxide with other chemicals, especially acids or bases.
Appropriate disposal methods for hydrogen peroxide and its decomposition products include:
- Diluting hydrogen peroxide with water and pouring it down the drain.
- Neutralizing hydrogen peroxide with a reducing agent, such as sodium thiosulfate.
Experimental Investigation
To investigate the rate of hydrogen peroxide decomposition, an experiment can be designed as follows:
| Variable | Value |
|---|---|
| Temperature | 25°C |
| pH | 7 |
| Catalyst | None |
| Hydrogen peroxide concentration | 1 M |
| Time | 0-60 minutes |
The experiment can be conducted by measuring the volume of oxygen gas produced over time. The data can then be analyzed to determine the rate of decomposition.
Future Directions, Hydrogen peroxide decomposes spontaneously to yield water and oxygen
Potential areas for further research on hydrogen peroxide decomposition include:
- Developing new catalysts to improve the efficiency and safety of decomposition processes.
- Exploring the use of hydrogen peroxide decomposition in emerging fields, such as energy storage and environmental remediation.
Question Bank
What is the chemical equation for hydrogen peroxide decomposition?
2H 2O 2→ 2H 2O + O 2
What are the physical and chemical changes that occur during hydrogen peroxide decomposition?
The physical change is the formation of oxygen gas bubbles. The chemical change is the conversion of hydrogen peroxide into water and oxygen.
What factors influence the rate of hydrogen peroxide decomposition?
Temperature, concentration, and the presence of catalysts.