Polarity and intermolecular forces gizmo assessment answers – Embark on a scientific expedition into the realm of polarity and intermolecular forces, as we delve into the Gizmo assessment and its profound implications. This comprehensive exploration unravels the intricacies of molecular interactions, revealing their profound influence on the properties and behavior of substances.
Delving into the depths of polarity, we uncover its pivotal role in shaping intermolecular forces. From the subtle nuances of dipole-dipole interactions to the captivating dance of hydrogen bonding, we witness how these forces orchestrate the physical characteristics of matter.
Polarity and Intermolecular Forces

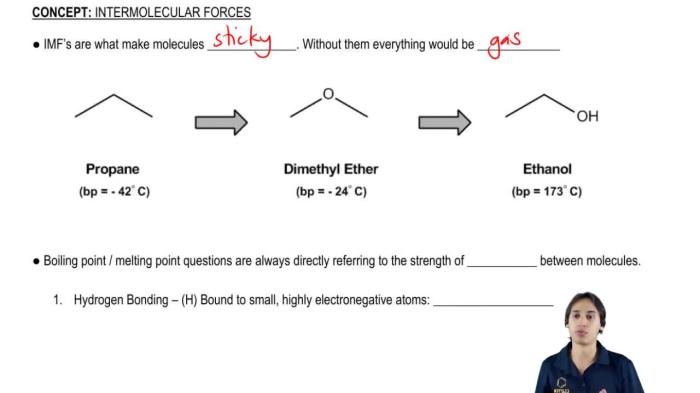
Polarity refers to the separation of electric charge within a molecule, resulting in a positive end and a negative end. It arises due to differences in electronegativity, which is the ability of an atom to attract electrons. Polarity influences intermolecular forces, which are the attractive forces between molecules.Polar
molecules have a permanent dipole moment, meaning they have a net positive or negative charge. Nonpolar molecules have no permanent dipole moment and are electrically neutral. Intermolecular forces are stronger between polar molecules than between nonpolar molecules.The types of intermolecular forces include:
-
-*Dipole-dipole interactions
Occur between polar molecules with permanent dipole moments. The positive end of one molecule attracts the negative end of another molecule.
-*Hydrogen bonding
A special type of dipole-dipole interaction that occurs between molecules containing hydrogen bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine).
-*London dispersion forces
Occur between all molecules, including nonpolar molecules. They arise from the temporary, instantaneous polarities that occur due to the movement of electrons within the molecule.
Gizmo Assessment Answers, Polarity and intermolecular forces gizmo assessment answers
The Gizmo assessment is an interactive tool that allows students to explore the concepts of polarity and intermolecular forces. It provides simulations and activities that help students visualize and understand these concepts.The results of the Gizmo assessment can be interpreted by comparing the student’s responses to the expected answers.
The assessment provides feedback on the student’s understanding of the material and identifies areas where they may need additional support.Tips for improving performance on the Gizmo assessment include:
- Reviewing the relevant concepts before taking the assessment.
- Paying attention to the instructions and following them carefully.
- Using the simulations and activities to visualize and understand the concepts.
- Taking notes during the assessment to help remember the key points.
- Seeking help from a teacher or tutor if needed.
Intermolecular Forces and Properties

Intermolecular forces affect the physical properties of substances. Substances with stronger intermolecular forces have higher melting points and boiling points. This is because more energy is required to overcome the intermolecular forces and separate the molecules.Polarity also influences intermolecular forces and, therefore, the physical properties of substances.
Polar molecules have stronger intermolecular forces than nonpolar molecules, leading to higher melting points and boiling points.Examples of substances with different intermolecular forces and their properties:
-
-*Water (polar)
High melting point (0 °C) and boiling point (100 °C) due to strong hydrogen bonding.
-*Methane (nonpolar)
Low melting point (-182 °C) and boiling point (-161 °C) due to weak London dispersion forces.
-*Ethanol (polar)
Intermediate melting point (-114 °C) and boiling point (78 °C) due to moderate dipole-dipole interactions.
Applications of Intermolecular Forces: Polarity And Intermolecular Forces Gizmo Assessment Answers
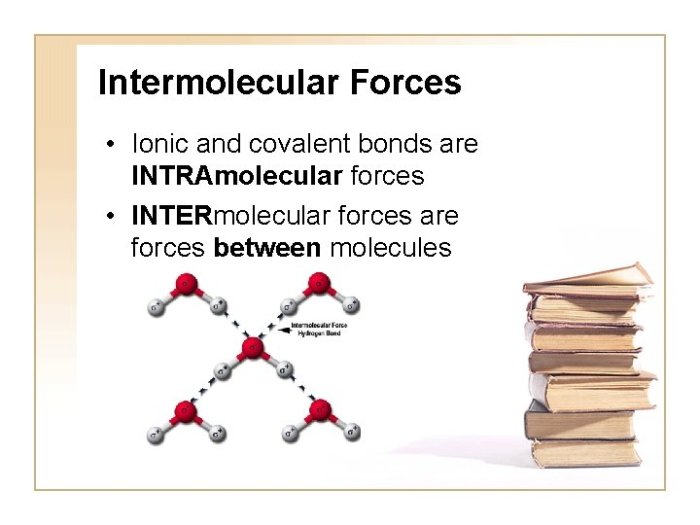
Intermolecular forces have numerous applications in everyday life, industry, and technology.Everyday life:
-
-*Adhesion
The ability of a substance to stick to another surface due to intermolecular forces, such as glue or tape.
-*Cohesion
The ability of a substance to stick to itself due to intermolecular forces, such as water droplets forming on a surface.
Industry:
-
-*Paints and coatings
Intermolecular forces determine the adhesion and durability of paints and coatings.
-*Pharmaceuticals
Intermolecular forces influence the solubility and bioavailability of drugs.
Technology:
-
-*Nanotechnology
Intermolecular forces are used to assemble and manipulate nanoparticles for various applications.
-*Sensors
Intermolecular forces are used in sensors to detect specific molecules or substances.
Biological systems:
-
-*Protein folding
Intermolecular forces play a crucial role in determining the structure and function of proteins.
-*Cell membranes
Intermolecular forces maintain the integrity and fluidity of cell membranes.
FAQ Summary
What is polarity?
Polarity refers to the uneven distribution of electrical charge within a molecule, resulting in the formation of a dipole with a positive end and a negative end.
How do intermolecular forces influence the properties of substances?
Intermolecular forces determine the strength of the interactions between molecules, affecting their physical properties such as melting point, boiling point, and solubility.
What is the purpose of the Gizmo assessment?
The Gizmo assessment provides an interactive platform for students to explore and understand the concepts of polarity and intermolecular forces through simulations and experiments.